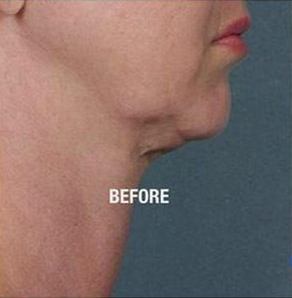
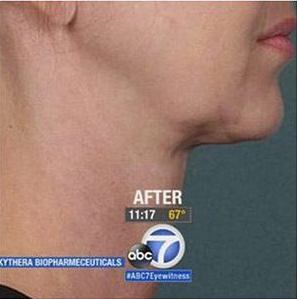
How Does Kybella® Work?
Submental fat is stored in fat cells that accumulate in the chin area. Kybella® has been described as a “fat-melting” compound, which is not completely accurate. It works by disrupting fat cell membranes, which causes cell destruction. This process is also known as cell “lysis.” When the membrane of the cell is destroyed, the fatty contents are released into the bloodstream and naturally metabolized by the body.